Seminar Series
Do · 12.06.2025
"Epigenetic determinants of genome stability for mammalian tissue", (project 1)
Speaker: Anna Eva Koch
Seminar Series
Do · 12.06.2025
"Epigenetic determinants of genome stability for mammalian tissue", (project 1)
Speaker: Anna Eva Koch
Seminar Series
Do · 26.06.2025
tba
Guest: Dr. Martijn S. Luijsterburg (Leiden University Medical Center)
Seminar Series
Do · 10.07.2025
"Mechanical-stress induced DNA damage and genome mechanoprotection in cellular and organismal homeostasis", (project 2)
Speaker: Tahira Aslan
Seminar Series
Do · 24.07.2025
tba
Guest: Dr. Daphne S. Cabianca (Helmholtz Munich)
Seminar Series
Do · 12.06.2025
"Epigenetic determinants of genome stability for mammalian tissue", (project 1)
This project will elucidate epigenetic mechanisms promoting genome instability when tissue homeostasis declines with age and under physiological stress. We discovered elevated G– quadruplex (G4) DNA secondary structure formation in mutated highly transcribed gene regulatory regions of cancer, suggesting dysregulated G4s as promoter of genome instability. We have and continue to gather evidence that dysregulated G4s emerge before cancer development in physiologically aged murine tissues and rapidly aged murine and human models. We hypothesize here that dysregulated G4 secondary structure formation may promote genome instability and heterostasis in aged and stress-induced rodent tissues. By exploiting our newly developed multiomics technology to jointly map epigenetically linked DNA breakage landscapes, beyond association, we will identify epigenetic mechanisms that critically drive persistent and/or elevated DNA breakage. To identify epigenetic regulators that promote an increase in epigenetically linked DNA breakage in tissues of our rodent models, we will use computational predictions and experimental associations of our multiomics data with existing data sets. We will use proximity ligation proteomics of tissue-derived nuclei to directly link regulatory factors to epigenetically linked DNA breakage and validate these findings by genetic loss-of-function in C. elegans.
Seminar Series
Do · 26.06.2025
title: tba
Seminar talk by Dr. Martijn S. Luijsterburg from Leiden University Medical Center.
Dr. Martijn S. Luijsterburg (Leiden University Medical Center) will present recent work on the molecular mechanisms underlying transcription-coupled DNA repair. His research combines genetic, genomic, and proteomic approaches to study how cells recognize and process DNA damage that interferes with transcription. The seminar will highlight new insights into the coordination between transcription, chromatin remodeling, and DNA repair pathways.
Seminar Series
Do · 10.07.2025
"Mechanical-stress induced DNA damage and genome mechanoprotection in cellular and organismal homeostasis", (project 2)
The nematode C. elegans is an excellent in vivo model system for DNA damage and repair responses. To understand the role of mechanical forces as physiological trigger of DNA damage in live animals, we will visualize and quantify relationships between nuclear deformation and DNA damage and analyze its functional consequences of organismal function, focusing on the highly dynamic C. elegans germline.
Seminar Series
Do · 24.07.2025
title: tba
Seminar talk by Dr. Daphne S. Cabianca from Helmholtz Zentrum, Munich.
Dr. Daphne S. Cabianca leads the Environment and Nuclear Organization group at Helmholtz Munich’s Institute of Functional Epigenetics. Her research focuses on how environmental cues—such as temperature or nutrient availability—reshape chromatin organization and gene expression. Using C. elegans as a model, her team combines CRISPR-based genome engineering, live imaging, and sequencing approaches to uncover how spatial chromatin dynamics contribute to cellular adaptation.

Tahira Aslan receives Oral Communication Prize of the Biochemical Society
The whole FOR5504 warmly congratulates Tahira Aslan on being awarded

Oral Communication Prize of the Biochemical Society for Tahira Aslan.
Congratulations to Tahira Aslan for the Oral Communication Prize of the Biochemical Society at the 92nd Harden Conference on Germ Cell Biology

Dr. Siyao Wang receives FEBS Excellence Award
The whole FOR5504 warmly congratulates Dr. Siyao Wang on being awarded the prestigious FEBS Excellence Award, recognizing outstanding contributions to molecular life sciences.

The whole FOR5504 warmly congratulates Dr. Siyao Wang on being awarded the prestigious FEBS Excellence Award, recognizing outstanding contributions to molecular life sciences.
FEBS Excellence Award for Siyao Wang
The father contributes nearly 80% of germline de novo mutations (DNMs) compared to the mother. While germline DNMs drive genetic diversity during evolution, they are also a fundamental cause of genetic disorders in offspring. Although it was written in the textbook that the paternal germline DNMs mainly arise from replication errors, emerging evidence suggests that many are due to errors in DNA damage repair. Aging is a common risk factor for paternal germline DNA damage due to increased intrinsic genotoxic attacks. Studies indicate that advanced paternal age is associated with a higher rate of germline DNMs and an increased incidence of schizophrenia and autism in progeny.
Wang’s group studies the molecular mechanism underlying the inheritance of paternal DNA damage response. By elucidating the mechanism, this work will provide insight into male-derived genetic diversity in evolution.
About the FEBS Excellence Award
The Federation of European Biochemical Societies (FEBS) launched the Excellence Awards scheme from 2021, it supports early-career group leaders in the FEBS area who have a scientific research track record of proven excellence. FEBS Excellence Awards provide funding of €100,000 for research equipment and consumables over a 3-year period.

"DNA Damage Tolerance: Physiological and Cancer Therapeutic Relevance"

DNA Damage Tolerance: Physiological and Cancer Therapeutic Relevance
Seminar talk by Dr. Heinz Jacobs from Netherlands Cancer Institute Antoni Van Leeuwenhoek.
DNA Damage Tolerance is Essential for Hematopoietic Stem Cell Maintenance and Mammalian Life
Stem cells are key players in central biological processes, such as tissue homeostasis, ageing, and cancer formation. Stem cell depend on genome maintenance to prevent disease formation. DNA damage tolerance (DDT) pathways enable DNA replication in the presence of replication impediments and are regulated by PCNAK164 ubiquitination and REV1. The failure to generate PcnaK164R/K164R;Rev1-/- deficient mice revealed DDT as essential for mammalian life. The compound mutation rendered hematopoietic stem cells (HSCs) and the hematopoietic precursors genetically unstable, instigating a pathological process where the associated HSC depletion culminated in a severe, embryonic-lethal anemia. Single cell RNA-sequencing of the remaining HSCs and progenitors identified CD24Ahigh and CD93low erythroid-biased progenitors (EBP) within the Lineage-, Sca1+, cKit- (LSK) population. In line, this subset was found to depend on the erythroid transcription factor Klf1. In conclusion, DDT is an essential activity within the DNA damage response network and in maintaining HSC fitness. By studying this system, we identified an erythroid-biased progenitor subset within the LSK compartment.
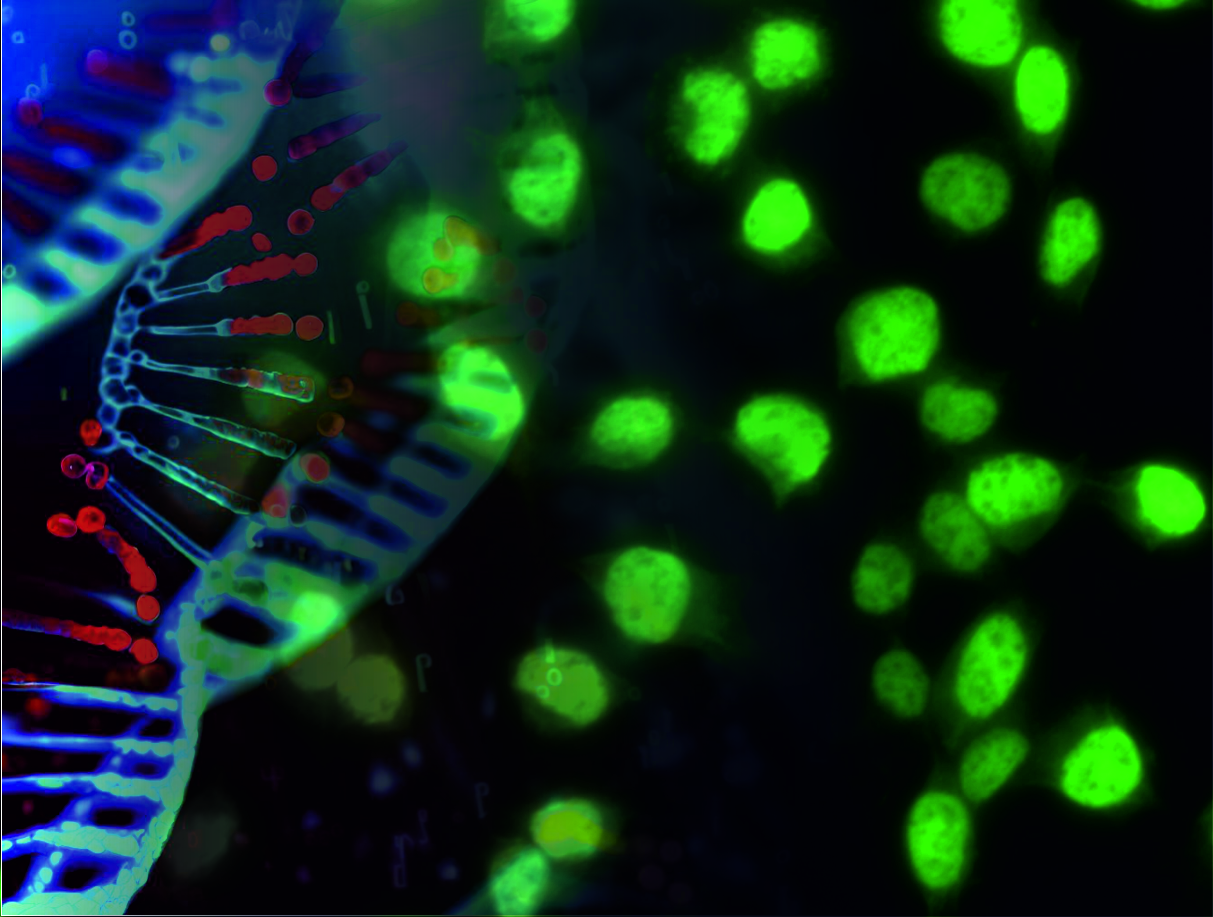
A damage driven transcription stress on aging and the effect of calorie restriction, (project 7)

A damage driven transcription stress on aging and the effect of calorie restriction, project 7
Joris investigates how chronic genome instability disrupts tissue homeostasis, focusing on transcriptional stress and its systemic impacts during aging. Using progeroid DNA-repair-deficient mouse models, he revealed that transcriptional stalling induces metabolic dysfunction and accelerates tissue degeneration, including neurodegeneration. His groundbreaking discovery of dietary restriction as a therapy shows it mitigates DNA damage, reprograms metabolism, and significantly extends lifespan, offering a translational approach to aging-related diseases.
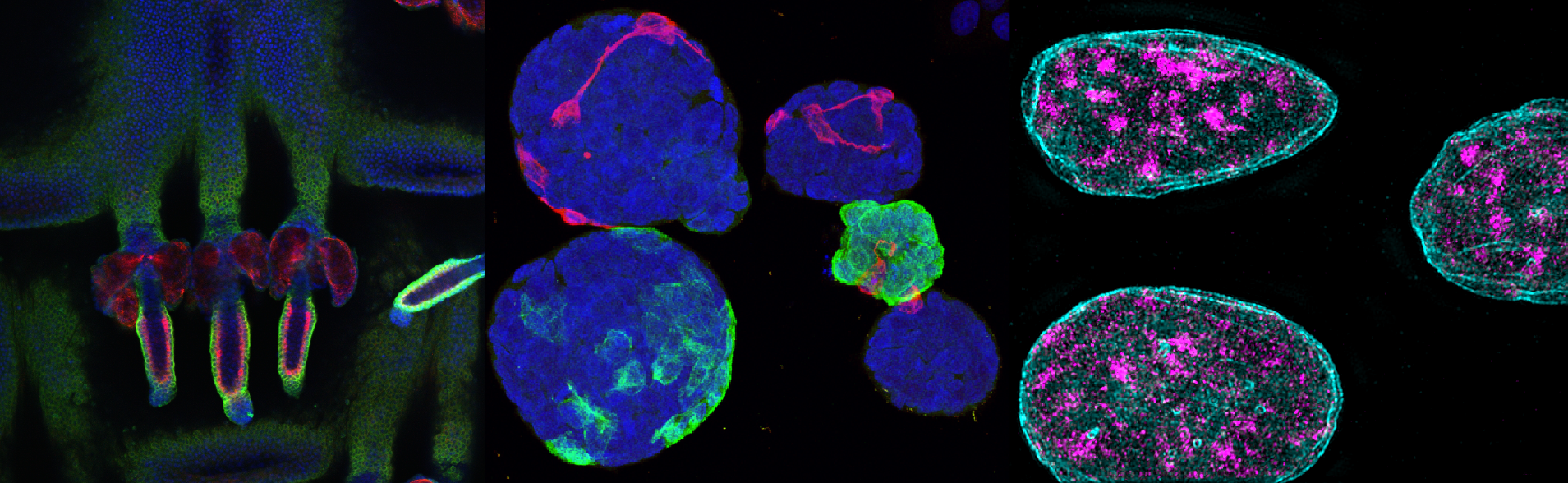
"Mechanical-stress induced DNA damage and genome mechanoprotection in cellular and organismal homeostasis", (project 2)

Sara and Anja are investigating how mechanical stress leads to DNA damage and affects genome mechanoprotection in cellular and organismal homeostasis. Their work focuses on uncovering the mechanisms through which physical forces trigger chromatin changes, replication stress, and DNA damage. This project aims to enhance our understanding of the role of mechanical stress in genome stability and its broader implications for tissue functionality.

Silent but deadly telomere interactions in cancer cells

"Silent but deadly telomere interactions in cancer cells"
Seminar talk in cooperation with the Cologne Graduate School of Ageing Research (CGA).
Dr. Roderick O'Sullivan's research at UPMC Pittsburgh focuses on understanding how telomeres, the protective caps at the ends of chromosomes, maintain genome stability. His lab investigates the proteins that regulate telomere structure and function, particularly within the Alternative Lengthening of Telomeres (ALT) pathway, which is implicated in certain cancers. By studying chromatin dynamics and DNA repair mechanisms at telomeres, O'Sullivan aims to uncover how telomere dysfunction can lead to genomic instability, a hallmark of cancer. His research also explores how modifications like poly-ADP-ribosylation impact telomere maintenance and cellular aging.

"Uncovering the link between mitochondrial dynamics and chromosome segregation", (affiliated group leader)

Uncovering the link between mitochondrial dynamics and chromosome segregation
Defects in mitotic progression can result in aneuploidy, cell death, and morphological abnormalities in daughter cells, often arising from improper organelle segregation, as demonstrated for the endoplasmic reticulum and Golgi. However, the impact of defective mitochondrial dynamics on mitotic progression, chromosome inheritance, and their broader physiological consequences remains largely overlooked, particularly in the context of mitochondrial diseases and aging. Our group is currently exploring the hypothesis that MTX2 plays a critical role in mitochondrial segregation during mitosis. Pathogenic mutations of MTX2, linked to a mitochondrial form of progeria, may disrupt this process, leading to mitotic errors that contribute to disease progression. Understanding these mechanisms could provide key insights into the interplay between mitochondrial dysfunction, mitotic defects, and aging-related disorders.

"The role of the DNA damage response in renal ciliopathies"

While humans and house mice develop kidney failure upon injury, the spiny mouse has a unique mechanism to repair kidney injury and prevent concomitant permanent kidney failure. My aim is to decipher the unique regenerative capacity by identifying the master switches which are repressed in humans and house mice by using tubuloids of spiny mice. The master switches can be targets in future regenerative medicine applications.

"Two cell cycle regulatory protein families: from functional studies to their use as novel diagnostic markers and therapeutic targets in cancer"

guest: Prof. Jörg Kobarg (University of Campinas)
Cancer is among the diseases that most kill humans and domestic animals. Depending on the tissue and cell type affected by mutations, a large number of sub-types of cancer can be differentiated. For a specific sub-type of cancer the molecular causes can be quite heterogeneous and for the majority of cases are poorly mapped. This represents both a major challenge as well as an opportunity in the field, since an intense study of the molecular causes can lead to new diagnostic and therapeutic strategies. Protein kinases in signaling pathways and regulatory proteins in general, are frequently affected by activating or inactivating mutations in cancer and therefore contribute to cancer transformation. We study two families of proteins with great potential of utility for both diagnostic and therapy in cancer. For the Nek family of serine/threonine protein kinases we focus on: Nek1,4,5,6,8, and 10, which are involved in the regulation of the cell cycle, mitosis, primary cilia function, DNA repair mechanisms and mitochondrial functions. The second family comprises the two human regulatory proteins, called HABP4 and SERBP1, that have roles in the regulation of transcription and gene expression, cell proliferation and the cell's responses to stress. Gene knock out studies and functional cellular and biochemical assays showed a new functional axis for the Nek kinases in the regulation of mitochondrial morphology and respiration. In case of the HABP1/SERBP1 regulatory proteins, molecular biology studies over the years point to a role in the regulation of mRNA stability or turn over. Novel data highlight the potential of both protein families for improved cancer diagnostics/prognostics, and in case of the Nek family members, also as potential novel target proteins for small molecular inhibitors, in cancer.
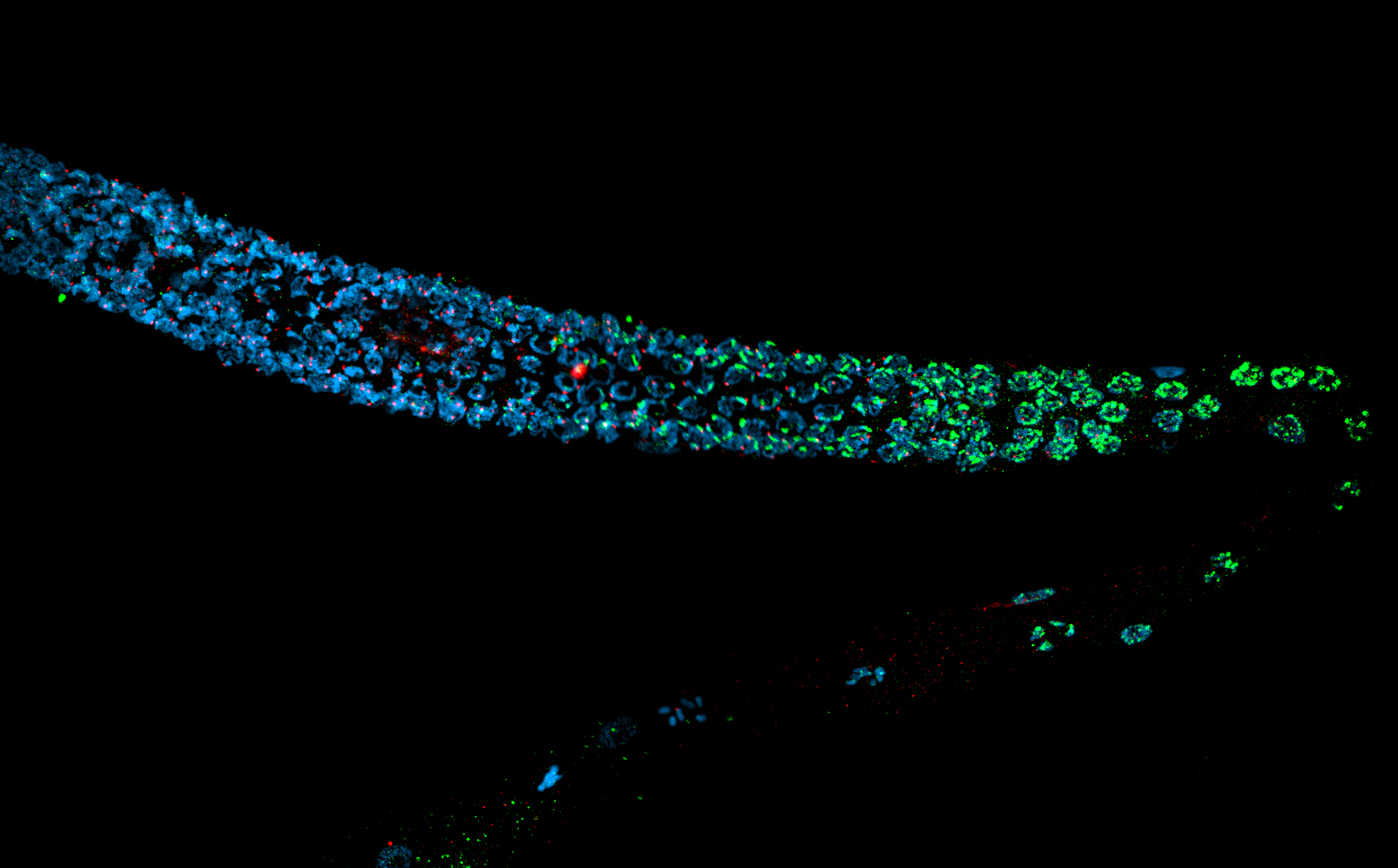
Epigenetic Regulation of Cellular Homeostasis Amid Transcription-Blocking DNA Damage During Development and Aging, project 3

Epigenetic Regulation of Cellular Homeostasis Amid Transcription-Blocking DNA Damage During Development and Aging, project 3
The research project focuses on how epigenetic mechanisms, particularly the deposition of H3K4me2 by the MLL/COMPASS complex, are regulated in response to transcription-blocking DNA damage. It investigates how this histone modification aids in the recovery of transcription elongation and the maintenance of cellular homeostasis during both development and aging. Using *C. elegans*, the study seeks to understand the physiological consequences of genome instability, particularly how impaired or enhanced deposition of H3K4me2 influences growth and longevity .

MD fellowship project reports

MD Fellowship Project Presentations
Giuseppe Oppedisano and Lukas Koch were awarded the FOR5504 MD Fellowship in November 2023, receiving a one-year stipend to dedicate full-time efforts to their individual research projects.
• Giuseppe Oppedisano conducted his research in the workgroup of Dr. Miguel Angel Alejandre Alcázar (Department of Pediatric and Adolescent Medicine). His project, titled “Interplay of Early Life Obesity, Genome Stability, and the Risk for Cardiopulmonary Diseases through Adipocyte-Secreted Extracellular Vesicles (EVs),” investigates how obesity in early life may influence genome stability and elevate risks for cardiopulmonary conditions via signals transmitted by EVs from fat cells.
• Lukas Koch worked in the group of Prof. Dr. Carien M. Niessen at CECAD. His project, “The Role of Cell Polarity in Mitochondrial Dynamics and Cell Fate in Field Cancerization and Squamous Cell Carcinoma Formation,” explores how disruptions in cell polarity impact mitochondrial behavior and contribute to the development of cancerous fields and squamous cell carcinoma.

"Repair of DNA double-strand by non-homologous end-joining"

Prof. Dr. Markus Löbrich (TU Darmstadt)
DNA double-strand breaks (DSBs) are major lesions induced by ionizing radiation and other DNA damaging agents. The main pathway for repairing DSBs in non-replicating cells is non-homologous end-joining (NHEJ). My talk will address two questions. First, I will talk about how NHEJ repairs DSBs which arise in transcribed genomic regions. This question is particularly important for long-living cells such as post-mitotic neurons which need to maintain gene functionality over decades. Second, I will address the question of how cells repair DSBs at very low damage levels. Super low damage levels represent the physiologically relevant situation and our findings suggests a new mechanism of cancer avoidance under such circumstances.

"tba", central project (Z) 1

"tba", (central project 1)
A computational platform for data support, integration and management
The Research Unit will generate large amounts of OMICS data. These data require powerful and sophisticated analyses. The Z1 project will support analysis, data integration within and among the individual projects as well as management to ensure efficient usage and reproducibility of the data among the Research Unit members. It will assist in planning OMICS experiments, for example, the design of technical and biological replicates and experimental blinding strategies. In addition, it will also provide training to members of the Research Unit to empower researchers to interrogate their own data and to properly document their work. The Z1 project leaders are experienced in generating and working with diverse and large-scale data types and are in a unique position to oversee and support this aspect of the Research Unit. The Z1 project will assist in the generation, selection, analysis, sustainable storage and usability of diverse OMICS data.

DNA Repair Meeting 2024 (DGDR)

Annual Meeting of the DGDR:
DNA Repair Meeting 2024
https://dgdr6.webnode.page/l/annual-dgdr-meeting/
September 25-27 | Cologne, Germany
Registration Deadline 30.6.24
Registration: :https://cms2.vcongress.de/dgdr-2024


joint FOR5504 retreat

"AGE-RELATED LOSS OF HETEROCHROMATIN IN OOCYTES AND GRANULOSA CELLS AND ITS ASSOCIATED FERTILITY OUTCOMES"

Guest: Dr. Michael Klutstein, The Chromatin and Aging Research Lab (CARL), The Hebrew University of Jerusalem

"Mechanism of genetic quality control in germ cells"

Guest: Dr. Volker Dötsch, Goethe-Universität Frankfurt
The survival rate of cancer patients is steadily increasing due to better and more efficient therapies. These advances in cancer therapy, however, create a new and increasing problem since treatment with chemotherapeutic drugs and radiation increases the risk of premature ovarian insufficiency (POI) for female cancer patients. While assisted reproductive technologies can address the problem of infertility, these measures cannot restore the hormonal functions pivotal for women’s health. Although recent advances in whole organ replacement could eventually offer an option to restore long-term hormone function and fertility in the future, a more detailed understanding of the molecular mechanisms of therapy-induced POI could identify targets for pharmacological prevention of POI during gonadotoxic therapies. Loss of the primordial follicle reserve is the most important cause of POI. TAp63a, a member of the p53 family, is the central transcription factor that upregulates the apoptotic program in oocytes. In resting primary oocytes it adopts an inactive state by forming a closed, only dimeric conformation. Detection of DNA damage activates the stress kinases CHk1 and CHk2 which leads to the phosphorylation of TAp63a which itself does not influence the conformation of TAp63a. Instead it recruits another kinase, CK1, which adds four more phosphate groups C-terminally to the CHk2 site. Electrostatic repulsion between the phosphate groups and a stretch of aspartic acids results in the destabilization of the closed dimeric state and the formation of the open and active tetramer. Measuring the phosphorylation kinetics has further shown that the individual CK1 phosphorylation events follow different kinetic regimes which is part of a kinetically encoded safety mechanism that triggers oocytes death only if a certain DNA damage level threshold is reached. This sophisticated system of monitoring the genetic integrity is evolutionary highly conserved but absent in male germ cells.

"Unravelling the role of RPA4 in genome stability using the naked mole-rat physiology."

"Unravelling the role of RPA4 in genome stability using the naked mole-rat physiology.", (FOR5504 - start-up funded postdoc)
The similarities between human replication protein A complex (RPA4) and naked mole rat (NMR) RPA4 (hgRPA) (62% protein identity) make the NMR an intriguing model for investigating aRPA function in vitro and in vivo. The RPA4 single-stranded DNA binding and winged-helix domains are also present in NMR (Ensembl and NCBI). Studying RPA4 in NMR could provide valuable insights into its contribution to genome stability, potentially shedding light on the remarkable healthspan and longevity of this unique rodent species.
Hence, with my independent project I aim to study three major points:

"ADP-ribosylation at the nuclear pore complex"

"ADP-ribosylation at the nuclear pore complex", (FOR5504-associated group leader)
'Talk less, say more'

trainer: Max Moenikes
In the pursuit of gender equality and equal opportunities in science, effective communication plays a pivotal role. This workshop is designed to empower individuals, with a particular focus on promoting and advancing women in science, by enhancing their rhetorical and communicative skills. In academic and professional settings, individuals often encounter speaking engagements ranging from team meetings and conferences to thesis presentations, funding applications, and teaching roles. While these opportunities can be exciting, they may also evoke feelings of stage fright and stress. However, with proper practice and preparation, anyone can confidently navigate these situations. In this seminar, we aim to cultivate essential rhetorical skills in a practical and engaging manner, recognizing the unique challenges faced by women in science. Our primary objective is to foster harmonious interactions among body language, voice, and content, enabling participants to authentically embody their intentions and garner approval from their audience.
The seminar curriculum is structured around the three fundamental pillars of rhetoric:
1) Body Language: Participants will learn to leverage posture, gestures, facial expressions, and eye contact for effective communication, creating a confident and impactful presence
2) Voice: Emphasis will be placed on techniques such as breathing, volume control, intonation and articulation, enabling participants to amplify their voices and convey their messages with clarity
3) Content: Strategies for structuring and planning speeches, as well as mastering argumentation techniques, will be explored, empowering participants to articulate their ideas persuasively

"Using CRISPR screens to map genetic determinants of radiation response."

Guest: Dr. Graeme Hewitt, CRUK RadNet Jr Group Leader

Aging clocks are based on random events / publication in ‘Nature Aging’ feat. Prof. Björn Schumacher and David Meyer
Scientists David Meyer and Professor Dr Björn Schumacher at CECAD, the Cluster of Excellence Cellular Stress Responses in Aging-Associated Diseases of the University of Cologne, have now discovered that aging clocks actually measure the increase in ...

News article about Aging clocks, featuring Prof. Dr. Schumacher and David Meyer.
Aging clocks can measure the biological age of humans with high precision. Biological age can be influenced by environmental factors such as smoking or diet, thus deviating from the chronological age that is calculated using the date of birth. The precision of these aging clocks suggests that the aging process follows a programme. Scientists David Meyer and Professor Dr Björn Schumacher at CECAD, the Cluster of Excellence Cellular Stress Responses in Aging-Associated Diseases of the University of Cologne, have now discovered that aging clocks actually measure the increase in stochastic changes in cells. The study ‘Aging clocks based on accumulating stochastic variation’ has been published in Nature Aging.
Read the full article: https://www.cecad.uni-koeln.de/outreach/news/article/how-aging-clocks-tick/

"Mechanical-stress induced DNA damage and genome mechanoprotection in cellular and organismal homeostasis", (project 2)

"Mechanical-stress induced DNA damage and genome mechanoprotection in cellular and organismal homeostasis", (project 2)
The nematode C. elegans is an excellent in vivo model system for DNA damage and repair responses. To understand the role of mechanical forces as physiological trigger of DNA damage in live animals, we will visualize and quantify relationships between nuclear deformation and DNA damage and analyze its functional consequences of organismal function, focusing on the highly dynamic C. elegans germline.
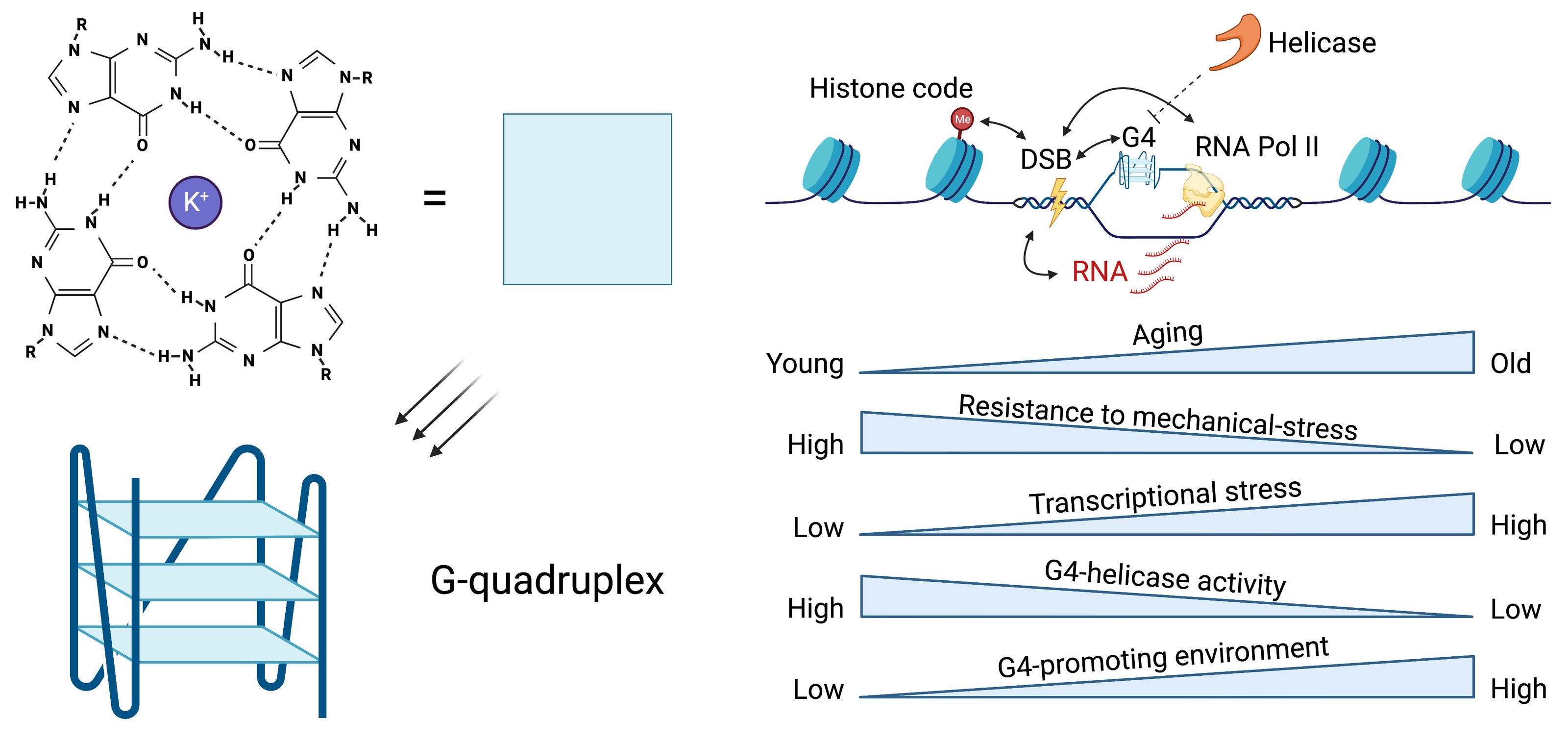
"Epigenetic determinants of genome stability for mammalian tissue", (project 1)

"Epigenetic determinants of genome stability for mammalian tissue", (project 1)
This project will elucidate epigenetic mechanisms promoting genome instability when tissue homeostasis declines with age and under physiological stress. We discovered elevated G– quadruplex (G4) DNA secondary structure formation in mutated highly transcribed gene regulatory regions of cancer, suggesting dysregulated G4s as promoter of genome instability. We have and continue to gather evidence that dysregulated G4s emerge before cancer development in physiologically aged murine tissues and rapidly aged murine and human models. We hypothesize here that dysregulated G4 secondary structure formation may promote genome instability and heterostasis in aged and stress-induced rodent tissues. By exploiting our newly developed multiomics technology to jointly map epigenetically linked DNA breakage landscapes, beyond association, we will identify epigenetic mechanisms that critically drive persistent and/or elevated DNA breakage. To identify epigenetic regulators that promote an increase in epigenetically linked DNA breakage in tissues of our rodent models, we will use computational predictions and experimental associations of our multiomics data with existing data sets. We will use proximity ligation proteomics of tissue-derived nuclei to directly link regulatory factors to epigenetically linked DNA breakage and validate these findings by genetic loss-of-function in C. elegans.

"The Ins and Outs of Cellular Senescence: Mechanistic, Therapeutic, and Diagnostic Insights"

Cellular senescence is beneficial during embryonic development, wound healing, and tumor suppression. Paradoxically, it is also a significant contributor to aging and age-related diseases, including cancer. As such, research on therapeutic strategies targeting senescence to improve health span has gained enormous momentum in recent years. However, discovering therapeutic targets for such therapies is still challenging, as our understanding of the molecular mechanisms that execute and maintain the senescence phenotype is incomplete. This seminar will delve into recent breakthroughs in deciphering a major senescence clock and explore this knowledge's exciting therapeutic, prognostic, and diagnostic potential.
"Metabolic deregulation, genome instability, and the progression of chronic kidney disease", (project 8)

"Metabolic deregulation, genome instability, and the progression of chronic kidney disease", (project 8)
The major aim of this research proposal is to unravel the interplay of genome instability, epigenetic alterations, and metabolic deregulation in CKD pathogenesis. Kidney function depends on the bulk filtration of large volumes of water and small solutes to clear potential toxins that are derived from intracellular metabolism and gastrointestinal microbial metabolism, as well as to maintain salt and water and acid-base homeostasis. Even slight losses of kidney filtration function cause profound metabolic changes in the organism which may explain elevated risk for CKD progression, cardiovascular complications, and other comorbidities. Based on two well-established mouse models that show a CKD phenotype, we will provide mechanistic insight into genome instability as a driver of kidney disease progression and characterize metabolic, transcriptomic, and epigenetic alterations caused by DNA damage in the kidney. Moreover, we will analyze the effects of metabolic interventions on disease progression, with the ultimate goal to develop strategies for future clinical applications.
"Genome Instability Syndromes as a Toolbox to Unravel Novel DNA Repair Pathways", (project 5)

We aim to explore the causes and consequences of deregulated DNA repair to explain important phenotypes observed in our novel human genome instability syndromes. Importantly, these insights will serve as a blueprint to understand the intricate relationship between the DDR, protein homeostasis and neurodegeneration.

Unconscious Bias in Academia for PhD-students/Postdocs

In this Workshop you will get the opportunity to deal with problems of social perception and
its diverse biases. Based on bias research, we will explore consequences for academic
careers and cooperation with a focus on equal opportunities. You can put your own
perception to the test and discuss tools for dealing professionally with implicit bias as well as
more explicit forms of prejudice.

Unconscious Bias in Academia for Principal Investigations

In this Workshop you will get the opportunity to deal with problems of social perception and
its diverse biases. Based on bias research, we will explore consequences for academic
careers and cooperation with a focus on equal opportunities. You can put your own
perception to the test and discuss tools for dealing professionally with implicit bias as well as
more explicit forms of prejudice.

"The genetic basis and pleiotropic nature of human physical traits"

There are substantial within and between population variations in human physical traits (e.g. skin and hair morphology, cranial and facial features). Comparing to diseases, physical traits receive far less attention and are largely understudied. For more than 10 years, our team has been systematically studying human physical traits, aiming to elucidate the genetic factors underlying the within and between population variations, and to reveal the developmental and evolutionary mechanisms. We further investigate the association between human physical traits and diseases/wellness, exploring the mechanism of the pleiotropic effects, and constructing prediction models. In the future, we will also focus on the effects of environmental exposure factors on phenotypes and their interactions with the genetic factors. Our studies provide valuable examples of the human phenome research.
Website: http://www.picb.ac.cn/dermatogenomics
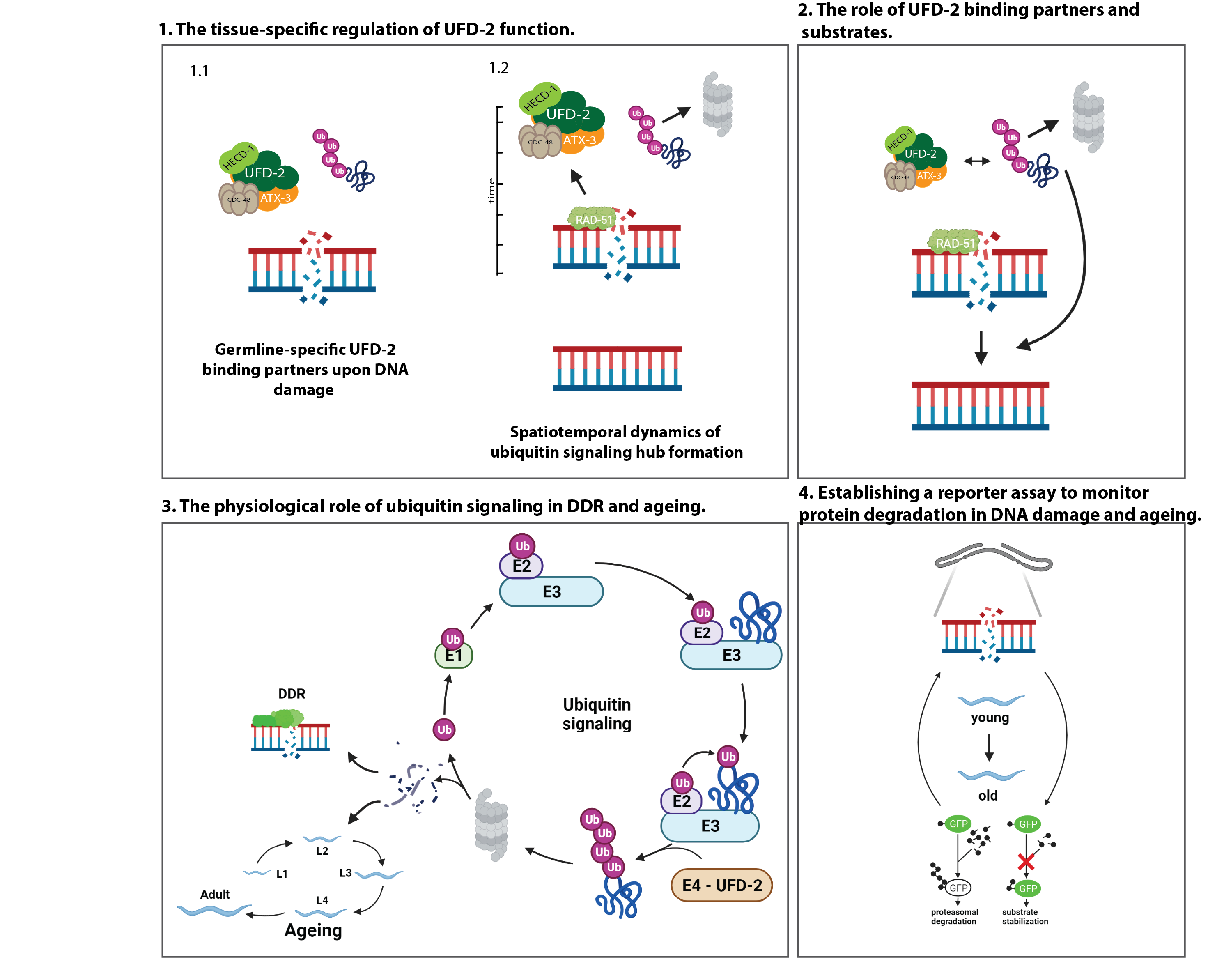
"Coordination of DNA Damage Response and Aging by Ubiquitin Signaling", project 4


The Science Breaker - Science meets Society
Mutations in the germline: How the mother repairs the father’s damaged genome

by Siyao Wang, David Meyer, Björn Schumacher
Germline mutations can have a severe impact on genetic diseases, genome evolution and the fate of a species. The vast majority of inheritable mutations are passed on by the paternal genome. We discovered how paternal DNA damage is repaired by maternal repair leading to the inheritance of structural variants.
Read the full article: https://www.thesciencebreaker.org/breaks/health-physiology/mutations-in-the-germline-how-the-mother-repairs-the-fathers-damaged-genome

"Understanding and modulating replicative senescence rates"

Replicative senescence refers to a stable cell cycle arrest that is induced through telomere shortening. Even in the presence of sufficient nutrients, senescent cells fail to proliferate. Senescence has both positive and negative effects. On the one side, senescence prevents unlimited cell proliferation and hence acts as a tumor suppressor; on the other side, the accumulation of senescent cells can contribute to aging phenotypes. It is therefore critical to control senescence rates to ensure that a non-pathological balance is achieved. I will show how the non-coding RNA, TERRA, through the formation of stable RNA-DNA hybrids, can regulate telomere dysfunction and senescence rates. In addition, I will show how gene expression profiling may be employed to predict novel regulators of senescence, which may provide additional avenues to modulate senescent cells. These results may be applicable in terms of both aging and cancer biology.
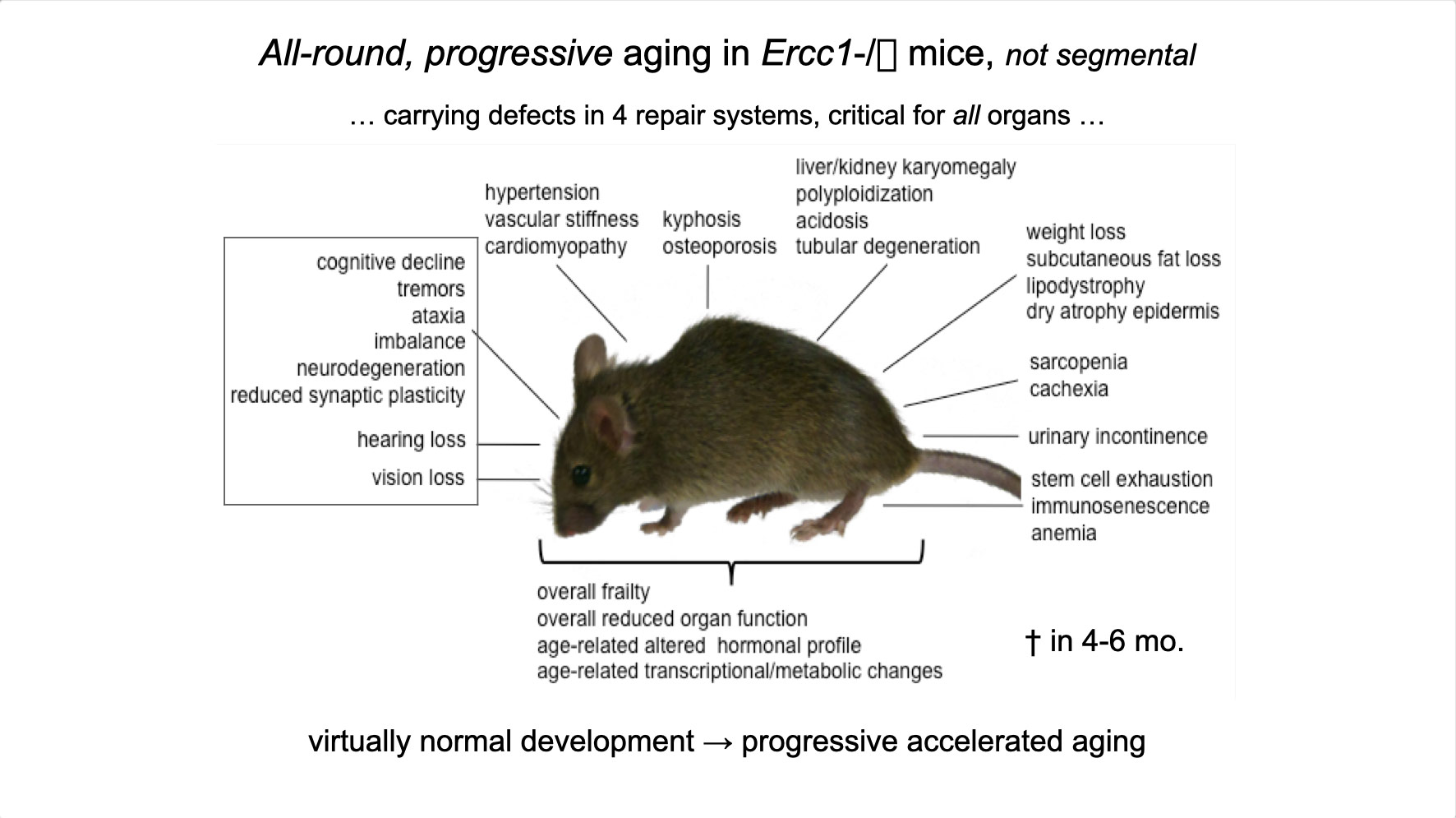
"The impact of DNA damage driven transcription stress on aging and the effect of calorie restriction", project 7


"What limits meiotic recombination?"
The capacity to reproduce and transmit genetic information is one of the few key features that define life. The Department of Chromosome Biology at the MPIPZ aims...

The capacity to reproduce and transmit genetic information is one of the few key features
that define life. The Department of Chromosome Biology at the MPIPZ aims to understand
how genetic information is transmitted and modified over generations.
The essence of heredity is meiosis, the special form of cell division that shuffles genetic
information at each generation and drives the evolution of all eukaryotes – from animals to
plants. The major focuses of the Department of Chromosome Biology are meiosis and
meiotic recombination. However, we also explore other key sources of genomic variation
such as mutations, genome rearrangements, holocentric centromeres and polyploidisation.

Joint Lab Seminar
To discuss relevant research and recent findings, the lab members of Dr. Wang, Prof. Wickström and Prof. Schumacher presented...

To discuss relevant research and recent findings, the lab members of Dr. Wang, Prof. Wickström and Prof. Schumacher presented and troubleshooted their work in this Joint-Lab-Seminar. Many insights, methods and new possible collaborations were discussed and determined.

Epigenetics Podcast feat. Prof. Schumacher
In this episode of the Epigenetics Podcast, we caught up with Björn Schumacher from ...

In this episode of the Epigenetics Podcast, we caught up with Björn Schumacher from the Institute for Genome Stability in Ageing and Disease at the University of Cologne and speaker of the FOR5504 to talk about his work on DNA damage in longevity and ageing.
In this episode Björn Schumacher discusses his research on DNA repair and its impact on ageing. We explore his insights on the effects of DNA damage on transcription, the importance of studying development, and the role of histone modifications. We also discuss paternal DNA damage inheritance and the DREAM complex as a master regulator of DNA repair. The lab’s goal is to enhance somatic DNA repair for healthier ageing and disease prevention.
Listen to his insights via https://www.activemotif.com/podcasts-bjorn-schumacher

First joint workshop of the DFG Research Unit ‘FOR5504’ and the NIA Program Project DNA Repair, Mutations, and Cellular Aging
Two outstanding research consortia from Germany and the United States focusing on the causes and consequences of genome instability and DNA repair in the aging process...

Two outstanding research consortia from Germany and the United States focusing on the causes and consequences of genome instability and DNA repair in the aging process held a joint workshop to exchange scientific concepts, discuss their latest research and develop future perspectives for the field.
The newly formed, DFG-funded FOR5504 ‘Physiological causes and consequences of genome instability’ with its speaker Prof. Björn Schumacher and vise-speaker Dr. Stephanie Panier presented groundbreaking perspectives and hypotheses to interdisciplinary investigate why physiological constrains posed on the genome stability, mechanical stress, metabolic factors, and constant infliction of DNA damage ultimately impacts tissue functionality and homeostasis.
Three invited keynote speakers provided overviews and perspectives during the workshop. Dr. Laura Niedernhofer (University of Minnesota) described her path of drug testing on senescent cells for the ability to prevent mortality in aged cells and the role of immune cells in aging. Dr. George Garinis (IMBB-FORTH) showed the latest insight into how DNA damage impacts immunometabolism and how distinct DNA repair mechanisms are linked to developmental gene expression programs in mammals. Dr. Björn Schwer (UCSF) shed light on how DNA repair and genome maintenance processes affect brain aging.
The long-standing National Institute on Aging, NIA, Program Project ‘DNA repair, mutations and cellular aging’ with their prominent Principal Investigators shared their latest fascinating findings in the field of DNA repair and aging research and, in a dedicated presentation by Jan Vig’s lab, state-of-the-art techniques to study somatic mutations were comprehensible depicted. All speakers and the audience enjoyed subsequent fruitful discussions and continued sharing their expertise and ideas even throughout the evening program.
FOR 5504
Universitätsklinik Köln
CECAD Research Center
Joseph-Stelzmann-Str. 26
50931 Köln
Tel. +49 (0)221 478 84198
simon.uszkoreit@uk-koeln.de

FOR5504 hat repostet Björn Schumacher
Huge congrats to @Meyer_DH for receiving the Best Poster award @ARDD_Meeting 2024. Well deserved for his outstanding work @CGA_age @UniCologne @CECAD_ 🥳🎇🎆
FOR5504
After a productive seminar day, we’re wrapping up with insightful scientific discussions and some great networking! #ResearchCommunity #Networking #Science
FOR5504
We are happy and glad to have our first joint FOR5504 retreat and host our labs at KSI Siegburg. Let's talk about genome stability 🧬 and enjoy science 👩🔬!
FOR5504 hat repostet Björn Schumacher
🔥🔥🔥my 💯 paper is out, congrats 🎈🎉🍾 @Meyer_DH for this amazing story. Aging clocks tick by the noise: we can build aging clocks 🕰️ on accumulating stochastic variation! @CECAD_ @UniCologne @CGA_age @UKKoeln follow 🧵retweet & read now open access:
Aging clocks based on accumulating stochastic variation: https://t.co/x3sCZoYkKP
CECAD Cologne
🧬👶⏰🧓
Congrats to @CECAD_ Professor @schumacherbj and @Meyer_DH at @UniCologne, who discovered that #agingclocks, which measure the biological age of humans with high precision, are based on #random events!
Read more: https://cecad.uni-koeln.de/outreach/news/article/how-aging-clocks-tick/ …
@NatureAging #aging
cecad.uni-koeln.de
How aging clocks tick
Two scientists from the University of Cologne have discovered that aging clocks are based on random events / publication in ‘Nature Aging’: https://t.co/uuDowdp2Hs
CECAD Cologne hat repostet Björn Schumacher
🔥🔥🔥Our review article on 🧬🧬🧬Genome Instability and DNA Repair in Somatic and Reproductive Aging @spanier55 and @SiyaoWang1005 @CECAD_ @MPIAGE @imbmainz @UKKoeln @UKKoeln
annualreviews.org
Genome Instability and DNA Repair in Somatic and Reproductive Aging
Genetic material is constantly subjected to genotoxic insults and is critically dependent on DNA repair. Genome maintenance mechanisms differ in somatic and germ cells as the soma only requires...: https://t.co/b9eJIYZL3W
CECAD Cologne hat repostet Universität zu Köln
Professor Dr Björn Schumacher, Institute for Genome Stability at CECAD, together with researchers from Frankfurt, Dublin (Ireland) and Singapore, receives the prestigious ERC Synergy Grant.‘BATPROJECT’ is to be financed with a total of 11.9 million euros ➡️https://uni.koeln/TRMG3
CECAD Cologne hat repostet Björn Schumacher
Our DREAM paper is out: We found the first master regulator of DNA repair capacities! Read and re-tweet now: The DREAM complex functions as conserved master regulator of somatic DNA repair capacities, @CECAD_ @UKKoeln @UniCologne
nature.com: https://t.co/rVMe4rAxaf