Thorsten Hoppe
Coordination of DNA damage response and aging by ubiquitin signaling
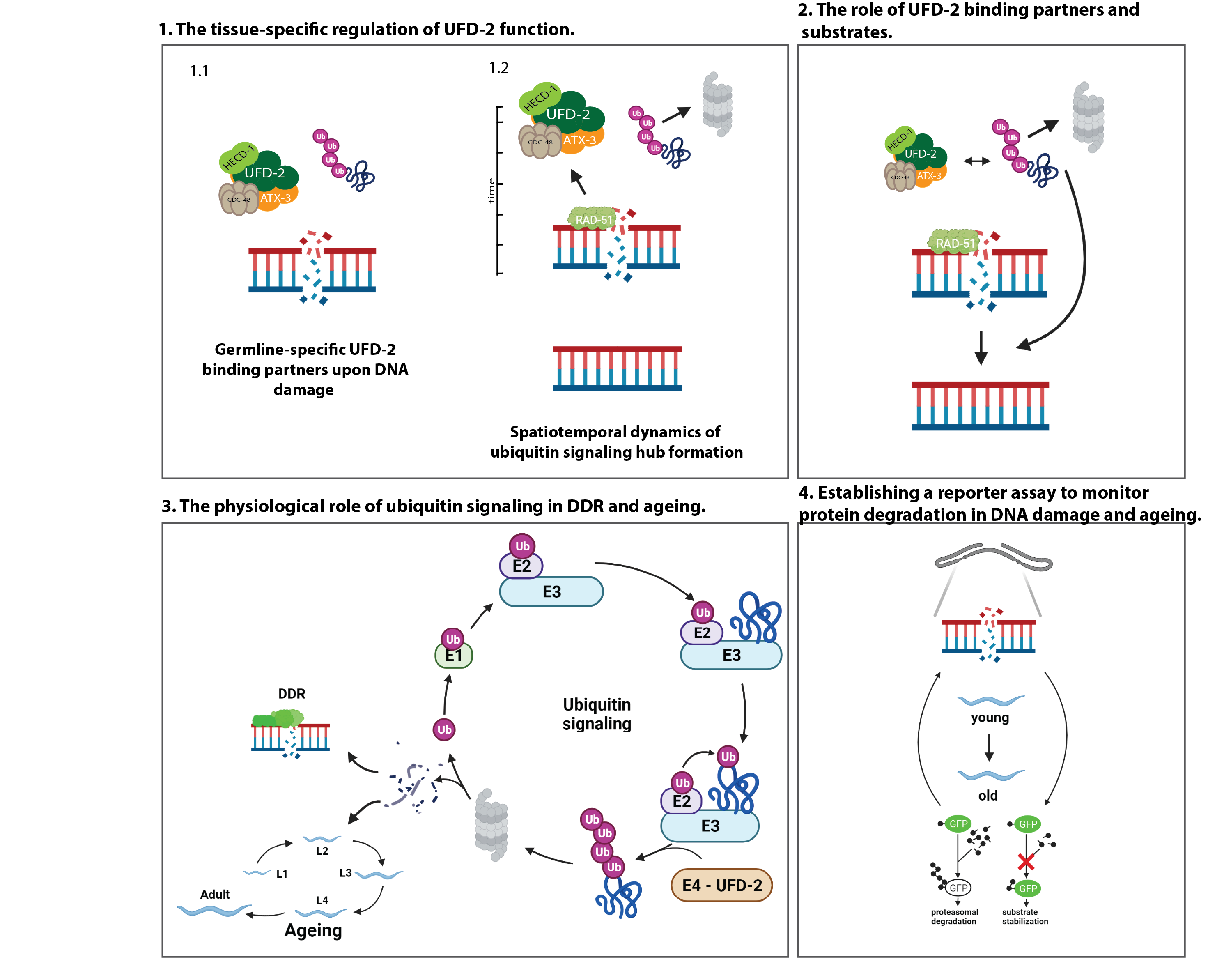
Repair of DNA double-strand breaks (DSBs) is tightly regulated by ubiquitin modification. Ubiquitin-dependent proteolysis is known to decrease with age, correlating with increased aggregation of damaged proteins. In contrast, the physiological role of the ubiquitin/proteasome-system (UPS) in age-related DNA damage accumulation and genome instability remains largely unclear. Our recent work has uncovered a central role of the conserved ubiquitin ligase UFD-2 in DSB repair, which is important for germline function, fecundity, and lifespan in C. elegans. UFD-2 is a specialized enzyme that triggers proteolysis by converting a nondegradable ubiquitin signal into a signal destined for substrate turnover by the 26S proteasome. Following DNA damage, UFD-2 forms focal accumulations in the germline that persist until homologous recombination is complete, reflecting the intricate coordination between ubiquitin-dependent regulation and DSB repair. The UFD-2 foci also contain the ubiquitin-selective segregase CDC-48/p97 and the deubiquitylation enzyme ATX-3/Ataxin-3, both of which have conserved functions in chromatin-associated protein degradation, DSB repair, and longevity. The main goal of the proposed research is to understand the physiological role of ubiquitin signaling in genome stability during development and aging. The project will address and systematically analyze the spatiotemporal coordination of ubiquitin-dependent DSB repair: the tissue-specific regulation of UFD-2 function and the physiological role of ubiquitin signaling and DNA damage response (DDR) in aging. To this end, in vitro and in vivo ubiquitylation assays, biotinylation-based protein-protein interaction mapping will be performed in combination with large-scale proteomics, CRISPR/Cas9 gene editing, time-lapse microscopy, and lifespan measurements will be performed.
PROJECT RELATED PUBLICATIONS
Articles published by outlets with scientific quality assurance, book publications, and works accepted for publication but not yet published. (*co-corresponding author)
- Franz A, Valledor P, Ubieto-Capella P, Pilger D, Galarreta A, Lafarga V, Fernández-Llorente A, de la Vega-Barranco G, den Brave F, Hoppe T*, Fernandez-Capetillo O*, Lecona E* (2021) USP7 and VCPFAF1 define the SUMO/Ubiquitin landscape at the DNA replication fork. Cell Rep. 37:109819
- Albert MC, Brinkmann K, Pokrzywa W, Günther SD, Krönke M, Hoppe T*, Kashkar H* (2020) CHIP ubiquitylates NOXA and induces its lysosomal degradation in response to DNA damage. Cell Death Dis: 11: 740
- Ackermann L, Schell M, Pokrzywa W, Kevei E, Gartner A, Schumacher B*, Hoppe T* (2016) E4 ligase specific ubiquitylation hubs coordinate DNA double strand break repair and apoptosis. Nat Struct Mol Biol. 23: 995-1002
- Franz A, Pirson PA, Pilger D, Halder S, Achuthankutty D, Kashkar H, Ramadan K, Hoppe T (2016) Chromatin-associated degradation is defined by UBXN-3/FAF1 to safeguard DNA replication fork progression. Nat Commun. 7: 10612
- Brinkmann K, Schell M, and Hoppe T*, Kashkar H (2015) Regulation of the DNA damage response by ubiquitin conjugation. Front. Genet. 6: 98
- Ermolaeva MA, Segref A, Dakhovnik A, Ou HL, Schneider JI, Utermöhlen O, Hoppe T, Schumacher B (2013) DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501: 416-420
- Dantuma NP* & Hoppe T* (2012) Growing sphere of influence: Cdc48/p97 orchestrates ubiquitin-dependent extraction from chromatin. Trends Cell Biol. 22: 483–491
- Acs K, Luijsterburg MS, Ackermann L, Salomons FA, Hoppe T, Dantuma NP (2011) The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat Struct Mol Biol. 18: 1345-1350
- Franz A, Orth M, Pirson PA, Sonneville R, Blow JJ, Gartner A, Stemmann O, Hoppe T (2011) CDC-48/p97 coordinates CDT-1 degradation with GINS chromatin dissociation to ensure faithful DNA replication. Mol Cell. 44: 85-96
- 10.Kuhlbrodt K, Janiesch PC, Kevei E, Segref A, Barikbin R, and Hoppe T (2011) The Machado-Joseph disease deubiquitylase ATX-3 couples longevity and proteostasis. Nat. Cell Biol. 13: 273-281

